Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ouchi, Kazuki
Hosha Kagaku, (49), p.3 - 7, 2024/03
I introduce the elucidation of the deposition following the oxidation state of uranium and the electrochemical behavior of uranium(IV) chloride in an ionic liquid-organic mixture, as a basic study of in-solution reactions. In addition, I introduce the development of separation methods for actinides using a microchemical chip and polyacrylamide gel electrophoresis, as an applied study for quantitative analytical methods for small amounts of samples.
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Solvent Extraction Research and Development, Japan, 31(1), p.1 - 11, 2024/00
A demonstration test was performed to separate minor actinides (MA; Am and Cm) by  -hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm
-hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm in
in  -dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were
-dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were  99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
Sato, Takumi; Otobe, Haruyoshi; Morishita, Kazuki; Marufuji, Takato; Ishikawa, Takashi; Fujishima, Tadatsune; Nakano, Tomoyuki
JAEA-Technology 2023-016, 41 Pages, 2023/09
This report summarizes the results of the stabilization treatments of post-experiment nuclear materials in Plutonium Fuel Research Facility (PFRF) from August 2018 to March 2021. Based on the management standards for nuclear materials enacted after the contamination accident that occurred at PFRF on June 6, 2017, the post-experiment nuclear materials containing plutonium (Pu): samples mixed with organic substances that cause an increase in internal pressure due to radiolysis (including X-ray diffraction samples mixed with epoxy resin and plutonium powder which caused contamination accidents), carbides and nitrides samples which is reactive in air, and chloride samples which may cause corrosion of storage containers, were selected as targets of the stabilization. The samples containing organic materials, carbides and nitrides were heated in an air flow at 650  C and 950
C and 950  C for 2 hours respectively to remove organic materials and convert uranium (U) and Pu into oxides. U and Pu chlorides in LiCl-KCl eutectic melt were reduced and extracted into liquid Cd metal by a reaction with lithium (Li) -cadmium (Cd) alloy and converted to U-Pu-Cd alloy at 500
C for 2 hours respectively to remove organic materials and convert uranium (U) and Pu into oxides. U and Pu chlorides in LiCl-KCl eutectic melt were reduced and extracted into liquid Cd metal by a reaction with lithium (Li) -cadmium (Cd) alloy and converted to U-Pu-Cd alloy at 500  C or higher. All of the samples were stabilized and stored at PFRF. We hope that the contents of this report will be utilized to consider methods for stabilizing post experiment nuclear materials at other nuclear fuel material usage facilities.
C or higher. All of the samples were stabilized and stored at PFRF. We hope that the contents of this report will be utilized to consider methods for stabilizing post experiment nuclear materials at other nuclear fuel material usage facilities.
Suzuki, Hideya*; Ban, Yasutoshi
Analytical Sciences, 39(8), p.1341 - 1348, 2023/08
Times Cited Count:2 Percentile:76.52(Chemistry, Analytical)The Japan Atomic Energy Agency (JAEA) has proposed the Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation (SELECT) process by solvent extraction as a new separation technology to recover minor actinides (MA) from high-level liquid waste (HLLW) produced by spent fuel reprocessing. The MA separation in the SELECT process comprises the batch recovery of MA and rare earths (RE) from HLLW, MA/RE separation, and Am/Cm separation. Three highly practical extractants are used in the MA separation. Furthermore, this flow configuration facilitates the preparation of nitric acid concentrations in the aqueous phase. However, the separation factor between Cm and Nd in the MA/RE separation is small (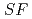
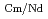 = 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
= 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
Kusaka, Ryoji
Bunko Kenkyu, 72(4), p.155 - 162, 2023/08
Vibrational sum frequency generation (VSFG) spectroscopy is an optical second-order nonlinear vibrational spectroscopy using ultrashort pulse lasers. Because VSFG spectroscopy is a unique and powerful tool for studying molecular structures of interfaces, it has been widely used in many research fields. However, there still undoubtedly remains some VSFG research areas that have not studied well, partly because VSFG measurements are not so easily performed in comparison with relatively general spectroscopy methods. This review presented recent applications of VSFG spectroscopy to two research topics: (1) chemical reactions on water surfaces, and (2) actinide chemistry.
Hayashi, Hirokazu; Tsubata, Yasuhiro; Sato, Takumi
Nihon Genshiryoku Gakkai Wabun Rombunshi (Internet), 22(3), p.97 - 107, 2023/08
The Japan Atomic Energy Agency has chosen nitride fuel as the first candidate for the transmutation of long-lived minor actinides (MA) using accelerator-driven systems (ADS). The pyrochemical method has been considered for reprocessing spent MA nitride fuels, because their decay heat should be very large for aqueous reprocessing. This study was conducted to investigate the effect of decay heat on the pyrochemical reprocessing of MA nitride fuels. On the basis of the estimated decay heats and the temperature limits of the materials that are to be handled in pyrochemical reprocessing, quantities adequate for handling in argon gas atmosphere were evaluated. From these considerations, we proposed that an electrorefiner with a diameter of 26 cm comprising 12 cadmium (Cd) cathodes with a diameter of 4 cm is suitable. On the basis of the size of the electrorefiner, the number necessary to reprocess spent MA fuels from 1 ADS in 200 days was evaluated to be 25. Furthermore, the amount of Cd-actinides (An) alloy to produce An nitrides by the nitridation-distillation combined reaction process was proposed to be about one-quarter that of Cd-An cathode material. The evaluated sizes and required numbers of equipment support the feasibility of pyrochemical reprocessing for MA nitride fuels.
 and O
and O in a reaction cell in triple quadrupole inductively coupled plasma mass spectrometry
in a reaction cell in triple quadrupole inductively coupled plasma mass spectrometryKazama, Hiroyuki; Konashi, Kenji*; Suzuki, Tatsuya*; Koyama, Shinichi; Maeda, Koji; Sekio, Yoshihiro; Onishi, Takashi; Abe, Chikage*; Shikamori, Yasuyuki*; Nagai, Yasuyoshi*
Journal of Analytical Atomic Spectrometry, 38(8), p.1676 - 1681, 2023/07
Times Cited Count:0 Percentile:0.02(Chemistry, Analytical) (M = Gd, Np) by the reaction of MN with Pd and chlorination of MPd
(M = Gd, Np) by the reaction of MN with Pd and chlorination of MPd using cadmium chloride
using cadmium chlorideHayashi, Hirokazu; Shibata, Hiroki; Sato, Takumi; Otobe, Haruyoshi
Journal of Radioanalytical and Nuclear Chemistry, 332(2), p.503 - 510, 2023/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)The formation of MPd (M = Gd, Np) by the reaction of MN with Pd at 1323 K in Ar gas flow was observed. Cubic AuCu
(M = Gd, Np) by the reaction of MN with Pd at 1323 K in Ar gas flow was observed. Cubic AuCu -type GdPd
-type GdPd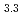 (
( = 0.4081
= 0.4081  0.0001 nm) and NpPd
0.0001 nm) and NpPd (
( = 0.4081
= 0.4081  0.0001 nm) were identified, respectively. The product obtained from the reaction of NpN with Pd contained additional phases including the hexagonal TiNi
0.0001 nm) were identified, respectively. The product obtained from the reaction of NpN with Pd contained additional phases including the hexagonal TiNi -type NpPd
-type NpPd . Chlorination of the MPd
. Chlorination of the MPd (M = Gd, Np) samples was accomplished by the solid-state reaction using cadmium chloride at 673 K in a dynamic vacuum. Pd-rich solid solution phase saturated with Cd and an intermetallic compound PdCd were obtained as by-products of MCl
(M = Gd, Np) samples was accomplished by the solid-state reaction using cadmium chloride at 673 K in a dynamic vacuum. Pd-rich solid solution phase saturated with Cd and an intermetallic compound PdCd were obtained as by-products of MCl formation.
formation.
Sato, Tetsuya; Nagame, Yuichiro*
Nihon Butsuri Gakkai-Shi, 78(2), p.64 - 72, 2023/02
The study of the chemistry of superheavy elements, which are located in the heavy extremes of the periodic table, has made considerable progress over the past 20 years, and new approaches based on various ideas have recently been developed. Research groups in Japan have also made significant contributions to the development of research on superheavy elements. Recently, notable results have been reported for the transactinide elements rutherfordium (element 104), dubnium (element 105), and seaborgium (element 106), and the heavy actinides with atomic numbers exceeding 100. The review will focus on the recent main results of these elements. This review outlines the main recent results and touches on future prospects.
Hirata, Sakiko*; Kusaka, Ryoji; Meiji, Shogo*; Tamekuni, Seita*; Okudera, Kosuke*; Hamada, Shoken*; Sakamoto, Chihiro*; Honda, Takumi*; Matsushita, Kosuke*; Muramatsu, Satoru*; et al.
Inorganic Chemistry, 62(1), p.474 - 486, 2023/01
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)Kusaka, Ryoji; Watanabe, Masayuki
Journal of Physical Chemistry Letters (Internet), 13(30), p.7065 - 7071, 2022/08
Times Cited Count:5 Percentile:70.33(Chemistry, Physical)Sato, Tetsuya; Nagame, Yuichiro*
Radiochimica Acta, 110(6-9), p.441 - 451, 2022/07
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)We describe our recent achievements in the effective production of low-energy ion-beams of the elements at the end of the actinide series, fermium (Fm, atomic number Z = 100), mendelevium (Md, Z = 101), nobelium (No, Z = 102), and lawrencium (Lr, Z = 103), using a surface ionization ion-source installed in the ISOL (Isotope Separator On-Line) at the Tandem accelerator facility of JAEA (Japan Atomic Energy Agency). Then the successful measurements of the first ionization potentials (IP ) of these elements with the ISOL setup are reviewed. The measured IP
) of these elements with the ISOL setup are reviewed. The measured IP values increased up to No via Fm and Md, while that of Lr was the lowest among the actinides. Based on the variation of the IP1 values of the heavy actinides with the atomic number in comparison with those of the heavy lanthanides, the results clearly demonstrated that the 5f orbitals are fully filled at No, and the actinide series ends with Lr. Furthermore, the IP
values increased up to No via Fm and Md, while that of Lr was the lowest among the actinides. Based on the variation of the IP1 values of the heavy actinides with the atomic number in comparison with those of the heavy lanthanides, the results clearly demonstrated that the 5f orbitals are fully filled at No, and the actinide series ends with Lr. Furthermore, the IP value of Lr provoked controversy over its position in the Periodic Table, so a short introduction to this issue is presented. The feasibility of the extension of chemical studies to still heavier elements with their ion-beams generated by ISOL is briefly discussed.
value of Lr provoked controversy over its position in the Periodic Table, so a short introduction to this issue is presented. The feasibility of the extension of chemical studies to still heavier elements with their ion-beams generated by ISOL is briefly discussed.
Toigawa, Tomohiro; Kumagai, Yuta; Yamashita, Shinichi*; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0502 (Internet), 2 Pages, 2022/04
This report summarizes the results obtained in FY2020 at the Electron Linac Facility of the University of Tokyo. The radiolysis process of  -hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
-hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
Sato, Nobuaki*; Kirishima, Akira*; Watanabe, Masayuki; Sasaki, Takayuki*; Uehara, Akihiro*; Takeda, Shino*; Kitatsuji, Yoshihiro; Otobe, Haruyoshi; Kobayashi, Taishi*
The Chemistry of Thorium, Plutonium and MA, 254 Pages, 2022/03
The chemistry of nuclear materials such as Thorium (Part 1) and Plutonium (Part 2) was described in relation from the fundamentals on solid chemistry and solution chemistry to the practicals on the experiment and evaluation method in detail. Minor actinides such as Neptunium, Americium, Curium and Protoactinium, was introduced the basics on the solid and solution chemistry.
Tanaka, Kazuya; Tani, Yukinori*; Kozai, Naofumi; Onuki, Toshihiko
Journal of Radioanalytical and Nuclear Chemistry, 331(2), p.1109 - 1114, 2022/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)We investigated the sorption of Pu(IV) on biogenic Mn oxide produced by Mn(II)-oxidizing fungus. The sorption of Pu(IV) on biogenic Mn oxide was similar to that of U(VI) and different from that of Th(IV), possibly due to oxidation of Pu(IV) to Pu(VI). When Pu(IV) was sorbed on hyphae only, it was desorbed into the solution phase over time. Pu(IV) could be solubilized by complexation with organic ligands secreted by fungal cells. In particular, Pu(IV) desorption was observed under circumneutral pH conditions.
Murakami, Tsuyoshi*; Hayashi, Hirokazu
Journal of Nuclear Materials, 558, p.153330_1 - 153330_7, 2022/01
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)Excess amounts of dissolution agents, CdCl and ZrCl
and ZrCl , are required to dissolve transuranium (TRU: Pu and minor actinides) nitrides into LiCl-KCl melts at the chemical dissolution step, which is the first step in the reprocessing of used nitride fuels. We propose an electrochemical process where the remaining Zr and Cd are recovered from the melts to be recycled as dissolution agents for the chemical dissolution step, leaving TRU in the melts. Since the initial concentration ratio of CdCl
, are required to dissolve transuranium (TRU: Pu and minor actinides) nitrides into LiCl-KCl melts at the chemical dissolution step, which is the first step in the reprocessing of used nitride fuels. We propose an electrochemical process where the remaining Zr and Cd are recovered from the melts to be recycled as dissolution agents for the chemical dissolution step, leaving TRU in the melts. Since the initial concentration ratio of CdCl /ZrCl
/ZrCl remaining in the melts would depend on the condition of the chemical dissolution step and would vary during the proposed electrochemical recovery process, electrochemical behaviors of Zr and Cd were investigated in LiCl-KCl melts with various concentration ratios of CdCl
remaining in the melts would depend on the condition of the chemical dissolution step and would vary during the proposed electrochemical recovery process, electrochemical behaviors of Zr and Cd were investigated in LiCl-KCl melts with various concentration ratios of CdCl /ZrCl
/ZrCl at 723 K to confirm the basic feasibility of the proposed process. Potentiostatic electrolysis was performed using a liquid Cd cathode at -1.05 V (vs. Ag/AgCl), which was a more positive potential than the redox potentials of TRU on the liquid Cd electrode. The obtained results showed that the current efficiency for recovering Zr and Cd from the melts was as high as 100% regardless of the CdCl
at 723 K to confirm the basic feasibility of the proposed process. Potentiostatic electrolysis was performed using a liquid Cd cathode at -1.05 V (vs. Ag/AgCl), which was a more positive potential than the redox potentials of TRU on the liquid Cd electrode. The obtained results showed that the current efficiency for recovering Zr and Cd from the melts was as high as 100% regardless of the CdCl /ZrCl
/ZrCl concentration ratio in the melts.
concentration ratio in the melts.
Sasa, Toshinobu
Kasokuki, 18(4), p.233 - 240, 2022/01
Lead bismuth eutectic alloy (LBE) is a promising option as a spallation target for accelerator-driven transmutation systems (ADS) to reduce the radiological toxicity from long-lived radioactive waste. LBE is a heavy metal and has suitable characteristics both as a spallation target and as a coolant for transmutation systems. However, LBE is also known as a highly corrosive with structural materials. In this paper, technological developments to overcome the issue, the latest research activities such as hightemperature operation and oxygen concentration control to ensure corrosion resistance, are introduced together with the outline of the target for ADS.
Kaneko, Masashi
Nihon Genshiryoku Gakkai-Shi ATOMO , 64(1), p.30 - 34, 2022/01
, 64(1), p.30 - 34, 2022/01
Partitioning of minor actinides from rare earths is one of the most important techniques to develop group separation of high-level radioactive liquid waste. In this issue, the results of prediction of separation performance between minor actinides and rare earths observed in solvent extraction and the separation mechanism by means of using density functional theory are explained.
Haraga, Tomoko; Saito, Shingo*
Bunseki Kagaku, 70(12), p.671 - 679, 2021/12
We developed highly sensitive capillary electrophoresis-laser-induced fluorescence detection methods for lanthanide (Ln) and actinide (An) ions with small sample volume and low emission of waste, by which the radiation risk can be minimized. Specifically, determination of Nd ion in spent nuclear fuel, effective separation between Am and Cm ion, and specific detection of UO
 in real radioactive samples were achieved by molecular design of fluorescence probes composed of an aminocarboxylate chelating moiety, a fluorophore and a spacer, and unique separation mode based on dynamic ternary complexation. We found that there are appropriate combination of probe and ternary complexation for detection and separation of each Ln and An ions. For example, acyclic and macrocyclic hexadentate is suitable for Ln
in real radioactive samples were achieved by molecular design of fluorescence probes composed of an aminocarboxylate chelating moiety, a fluorophore and a spacer, and unique separation mode based on dynamic ternary complexation. We found that there are appropriate combination of probe and ternary complexation for detection and separation of each Ln and An ions. For example, acyclic and macrocyclic hexadentate is suitable for Ln , Am
, Am and Cm
and Cm , and planer tetradentate with
, and planer tetradentate with  electron system is specific for UO
electron system is specific for UO
 , with ppt-sub ppt level detection.
, with ppt-sub ppt level detection.
Miyazaki, Yasunori; Sano, Yuichi
Hoshasen Kagaku (Internet), (112), p.27 - 32, 2021/11
no abstracts in English